Made Smarter Innovation CR&D Project Showcase
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Etiam dolor magna, commodo posuere lobortis at, suscipit in est. Suspendisse lacinia...
Built by Advanced Therapy Manufacturers for Advanced Therapy Manufacturers
With our extensive audit logging feature, you can have complete trust that everything that happens on the system is tracked & traceable, and every input and change in the manufacturing record is logged with the precise date and time as well as the identity of the recording user.
AutoloMATE® is designed to comply with guidance from UK MHRA, EU EMA & US FDA, going a step further through its use of ledger technology to ensure data immutability – records cannot be falsified and this can be demonstrated for every relevant item of data and transaction.
AutoloMATE® provides a set of features to enhance Quality Assurance and Control such as special control tasks accessible only by qualified individuals and e-signing of the audit log review. Our software supports raising quality events, facilitating review and resolution.
By automating key parts of the manufacturing process such as running calculations, transcribing data from BMRs, forms & external systems like LIMS, AutoloMATE® speeds up processes, eliminates errors & improves communication of data between key departments.
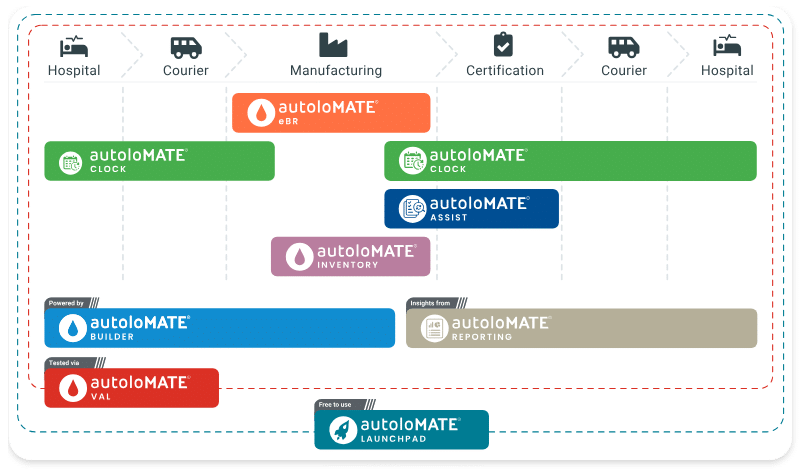
Autolomous provides manufacturers with ways to scale their operations, by deploying a fully integrated and digitised supply chain ecosystem.

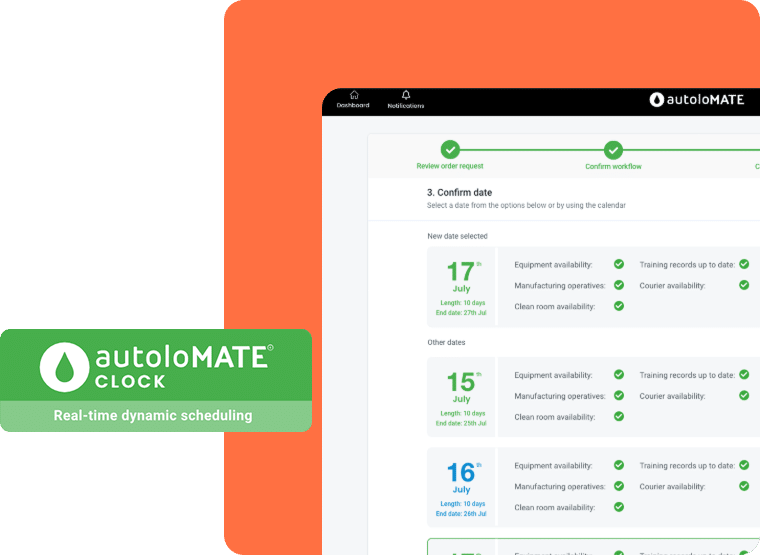
AutoloMATE CLOCK digitises all aspects of manufacturing scheduling to facilitate optimisation of manufacturing facility operations. By accounting for all critical data – such as clinicians schedule, equipment and clean room availability, courier interactions, CLOCK enables the scale-up and maximised utilisation of manufacturing resources while driving down cost-of-goods.

AutoloMATE eBMR, which is commercially available, digitises batch manufacturing records, eliminating the need for vast amounts of paper. It has been designed to provide agility in a controlled environment, and can be configured for all different types of advanced therapy workflows, including Autologous – CAR-T, TIL, DC vaccine, and Allogeneic NK, IPSC, MSC. Using real-time data verification, AutoloMATE eBMR can accelerate the product review and release process reducing the total time required from up to 36 hours to as little as 2.
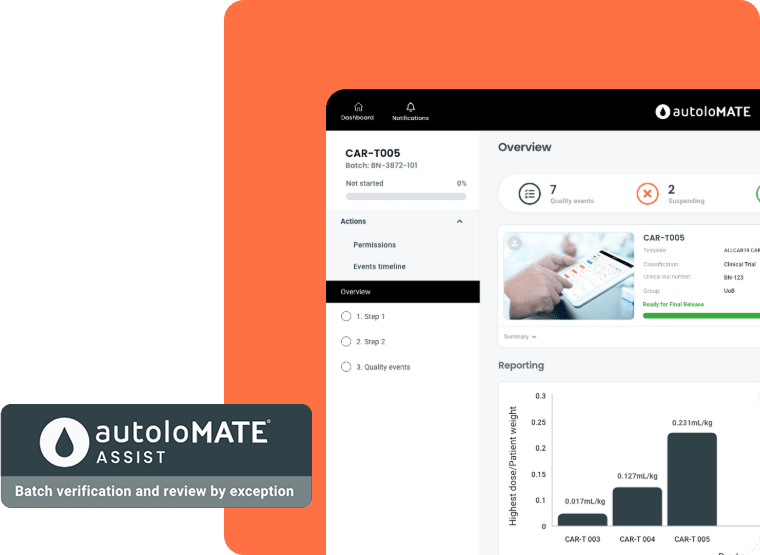
AutoloMATE Assist supports the batch verification of advanced therapies. Data collated on the AutoloMATE® platform via AutoloMATE eBMR and AutoloMATE CLOCK will be assessed against specifications defined in the Critical Quality Attributes and visually presented to quality stakeholders (QC, QA, QP) enabling them to carry out assessments based on “review by exception” methodologies. Controlling and reducing manual data operations increases the integrity of the data, while reducing the risks associated, together driving efficiency, throughput and delivery of advanced therapies to patients.
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Etiam dolor magna, commodo posuere lobortis at, suscipit in est. Suspendisse lacinia...
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Etiam dolor magna, commodo posuere lobortis at, suscipit in est. Suspendisse lacinia...
Lorem ipsum dolor sit amet, consectetur adipiscing elit. Etiam dolor magna, commodo posuere lobortis at, suscipit in est. Suspendisse lacinia...
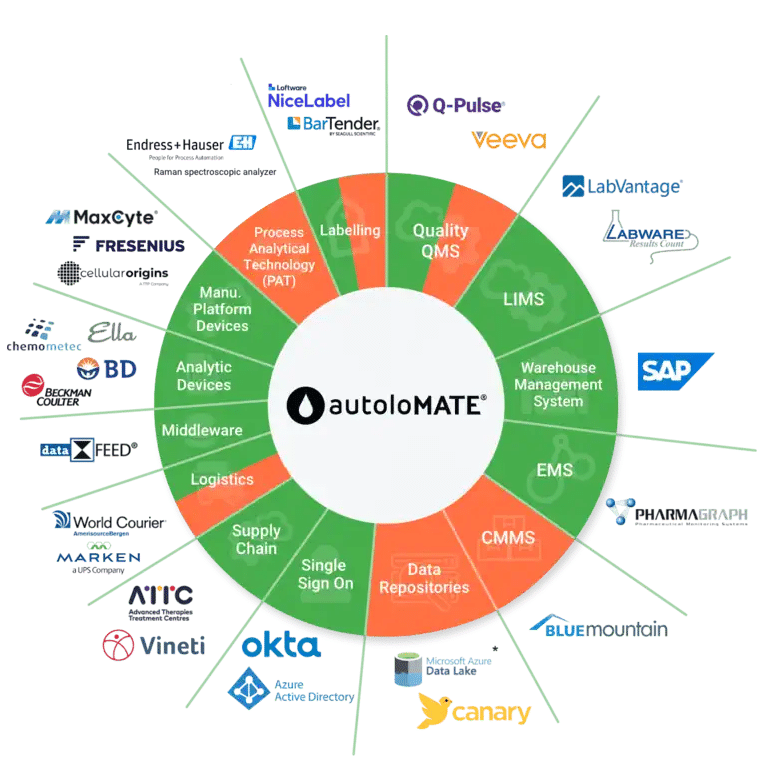
AutoloMATE® comes with an always expanding set of integrations to external devices and systems, ranging from Laboratory Information Management Systems (LIMS) and Quality Management Systems (QMS) to cell counters & flow cytometers. These integrations allow us to get data from systems and devices and put them to use in the manufacturing record, speeding up processes, eliminating transcription errors, and simplifying the use of version controlled documents such as standard operating procedures.
The eBR Builder will allow advanced therapy manufacturers to configure and edit eBMRs on the AutoloMATE® platform on their own. It will equip manufacturers with the resilience and agility required to adequately react to process changes while de-risking the time and cost of engaging the supplier.
The AutoloMATE Reporting module will unlock insights from within eBRs, allowing for trends, bottlenecks and learnings to be identified and leveraged to achieve manufacturing optimisation and scale-up.
Everything that happens within the AutoloMATE® platform will be tracked and available for review to our clients’ system administrators – from name changes to login / log out attempts, product records access, and more, providing an overall useful tool for investigative and record-keeping purposes.
User group segregation will allow CDMOs to keep data belonging to manufacturers who use their facilities fully segregated while using a single platform. Groups can also be used to separate users into different projects within the same company.
To delve further into what we do at Autolomous, contact us to request an info pack or to get more details.
